Absolute VS Nominal Filters
Basics of waterborne diseases
Bubble point definition
Chemical adsorption of carbon
Granular activated carbon fact sheet
How do carbon filter work?
Water Softener – How do they work? – FAQs
What is a filter Beta Ratio
What is Osmosis and Reverse Osmosis
Water Glossary
What is Ultrafiltration
What is Ultraviolet water treatment?
ACTIVATED CARBON ADSORPTION
Adsorption is a process where a solid (active carbon) is used for removing a soluble substance from the water. Activated carbon is produced specifically to achieve a very big internal surface (between 500 – 1500 m2/g). The big internal surface makes active carbon ideal for adsorption. Active carbon comes in Powder Activated Carbon (PAC) and Granular Activated Carbon (GAC). The GAC version is mostly used in water treatment, it can adsorb the following substances:
- Odor
- Taste
- VOC’s
- PCB’s
- Chlorine
- Trihalomethanes
- Yeasts
- Various fermentation products
- Adsorption of halogenated substance: I, Br, Cl, H en F
- Adsorption of organic, non-polar substances such as:
- BTEX
- Mineral oil
- (Chloride) phenol
- Poly aromatic hydrocarbons (PACs)
Non-polar substances (Substances which are non-soluble in water)
Examples from active carbon in different processes:
- Ground water purification
- The polishing of treated effluent
- Water purification for swimming pools
- The dechlorination of process water
PROCESS DESCRIPTION:
Water is pumped in a column which contains active carbon and then the water leaves the column through a draining system. The action of an active carbon column depends on the temperature and the nature of the substances. Water goes through the column constantly, which gives an accumulation of substances in the filter and therefore, needs to be replace periodically. A used filter can be regenerated in different ways, granular carbon can be regenerated easily by oxidizing the organic matter and the efficiency of the active carbon decreases by 5 – 10% 1). A small portion of the active carbon is destroyed during the regeneration process and must be replaced. If you work with different columns in series, you can be assured that you will not have a total exhaustion of your purification system.
DESCRIPTION OF ADSORPTION:
Molecules from gas or liquid phase will be attached (adsorbed, not absorbed) in a physical way to the carbon surface. The adsorption process takes place in three steps:
- Macro transport: The movement of organic material through the macro-pore system of the active carbon (macro-pore >50nm)
- Micro transport: The movement of organic material through the meso-pore and micro-pore system of the active carbon (micro-pore <2nm; meso-pore 2-50nm)
- Sorption: The physical attachment of organic material on the surface of active carbon in the meso-pores and micro-pores of the active carbon
The concentration of substance in the water determines the activity level of adsorption, the temperature and the polarity of the substance. A polar substance is a substance which is good soluble in water, cannot or is badly removed by active carbon, a non-polar substance can be removed totally by active carbon. Every kind of carbon has its own adsorption isotherm (see figure 1) and in the water treatment business this isotherm is definite by the function of Freundlich.
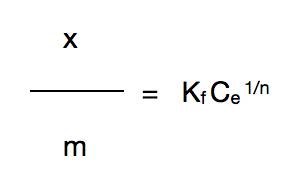
The function of Freundlich: x/m = adsorbed substance per gram active carbon
Kf, n = specific constants
Ce = concentration difference (between before and after)
The second curve from active carbon (see figure 2) shows a filter that is exhausted. Usually we place a UV-disinfections unit after the active carbon column.
WHAT IS THE DIFFERENCE BETWEEN ADSORPTION AND ABSORPTION?
When a substance is attached to a surface this is called adsorption, where the substance is attached to the internal surface of active carbon. When a substance is absorbed in a different medium it is called absorption, for example, when a gas is taken in a solution. it is called absorption.
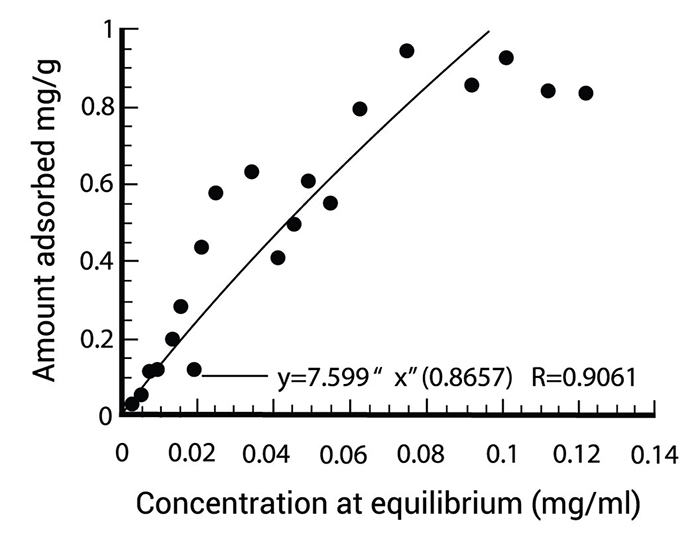
Figure 1 gives a specific adsorption isotherm for active carbon. On the horizontal axis you can find the concentration and on the vertical axis you can find the necessary quantity of carbon. You can use this kind of figures to optimize your column.
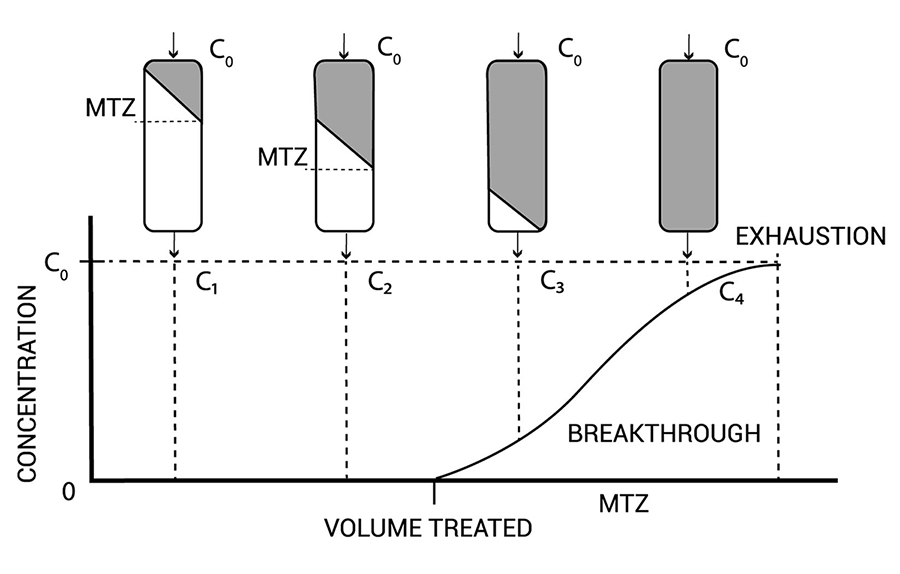
Figure 2 tells about the exhaustion during usage of your column. Point C3 the column starts to break through and near C4 your column is not purifying anymore. Between point C3 and C4 you need to regenerate your column.
Elements that influence the performance of active carbon in water:
- The higher the concentration, the higher the carbon consumption.
- Compounds with high molecular weight and low solubility are better absorbed.
- The pH of the waste stream. For example, acidic compounds are better removed at lower pH.
- Company of other organic compounds which will compete for the available adsorption sites.
We can classify some chemicals by their probability of being efficiently adsorbed by active carbon in water:
1.- Chemicals that have a very high probability of being adsorbed by active carbon:
| 2,4-D | Deisopropyltatrazine | Linuron |
| Alachlor | Desethylatrazine | Malathion |
| Aldrin | Demeton-O | MCPA |
| Anthracene | Di-n-butylphthalate | Mecoprop |
| Atrazine | 1,2-Dichlorobenzene | Metazachlor |
| Azinphos-ethyl | 1,3-Dichlorobenzene | 2-Methyl benzenamine |
| Bentazone | 1,4-Dichlorobenzene | Methyl naphthalene |
| Biphenil | 2,4-Dichlorocresol | 2-Methylbutane |
| 2,2-Bipyridine | 2,5-Dichlorophenol | Monuron |
| Bis(2-Ethylhexyl)Phthalate | 3,6-Dichlorophenol | Napthalene |
| Bromacil | 2,4-Dichlorophenoxy | Nitrobenzene |
| Bromodichloromethane | Dieldrin | m-Nitrophenol |
| p-Bromophenol | Diethylphthalate | o-Nitrophenol |
| Butylbenzene | 2,4-Dinitrocresol | p-Nitrophenol |
| Calcium Hypochloryte | 2,4-Dinitrotoluene | Ozone |
| Carbofuran | 2,6-Dinitrotoluene | Parathion |
| Chlorine | Diuron | Pentachlorophenol |
| Chlorine dioxide | Endosulfan | Propazine |
| Chlorobenzene | Endrin | Simazine |
| 4-Chloro-2-nitrotoluene | Ethylbenzene | Terbutryn |
| 2-Chlorophenol | Hezachlorobenzene | Tetrachloroethylene |
| Chlorotoluene | Hezachlorobutadiene | Triclopyr |
| Chrysene | Hexane | 1,3,5-Trimethylbenzene |
| m-Cresol | Isodrin | m-Xylene |
| Cyanazine | Isooctane | o-Xylene |
| Cyclohexane | Isoproturon | p-Xylene |
| DDT | Lindane | 2,4-Xylenol |
2.- Chemicals that have a high probability of being adsorbed by active carbon:
| Aniline | Dibromo-3-chloropropane | 1-Pentanol |
| Benzene | Dibromochloromethane | Phenol |
| Benzyl alcohol | 1,1-Dichloroethylene | Phenylalanine |
| Benzoic acid | cis-1,2- Dichloroethylene | o-Phthalic acid |
| Bis(2-chloroethyl) ether | trans-1,2- Dichloroethylene | Styrene |
| Bromodichloromethane | 1,2-Dichloropropane | 1,1,2,2-Tetrachloroethane |
| Bromoform | Ethylene | Toluene |
| Carbon tetrachloride | Hydroquinone | 1,1,1-Trichloroethane |
| 1-Chloropropane | Methyl Isobutyl Ketone | Trichloroethylene |
| Chlorotoluron | 4-Methylbenzenamine | Vinyl acetate |
3.- Chemicals that have a moderate probability of being adsorbed by active carbon*:
| Acetic acid | Dimethoate | Methionine |
| Acrylamide | Ethyl acetate | Methyl-tert-butyl ether |
| Chloroethane | Ethyl ether | Methyl ethyl ketone |
| Chloroform | Freon 11 | Pyridine |
| 1,1-Dichloroethane | Freon 113 | 1,1,2-Trichloroethane |
| 1,2-Dichloroethane | Freon 12 | Vinyl chloride |
| 1,3-Dichloropropene | Glyphosate | |
| Dikegulac | Imazypur |
*(For these chemicals active carbon is only effective in certain cases).
4.- Chemicals for which adsorption with active carbon is unlikely to be effective and may be viable in certain cases such as for low flow or concentrations:
| Acetone | Methylene chloride |
| Acetonitrile | 1-Propanol |
| Acrylonitrile | Propionitrile |
| Dimethylformaldehyde | Propylene |
| 1,4-Dioxane | Tetrahydrofuran |
| Isopropyl alcohol | Urea |
| Methyl chloride |
Elements that influence the performance of active carbon in air:
- Humidity: The lower the humidity, the better the adsorption capacity.
- Pressure: The higher the pressure, the better the adsorption capacity.
- Temperature: The lower the temperature, the better the adsorption capacity.
- Concentration: The higher the concentration, the higher the carbon consumption.
- Type of compound to be removed: In general compounds with a high molecular weight, lower vapor pressure/higher boiling point and high refractive index are better adsorbed.
